Metal passivation chemically treats metal to reduce its surface chemical reactivity. Reducing surface chemical reactivity helps inhibit corrosion in metal objects. In this article, you’ll learn about the benefits of metal passivation, the process, and alternatives.
Metal Passivation Benefits
Metal corrosion is a chemical phenomenon that occurs when an active metal alloy reacts in ambient conditions to become a more stable compound. Oxides and sulfides are the main corrosion compounds. For example, simple exposure to the humid air of iron can lead to a chemical reaction forming rust.
Fabrication processes, including machining and welding, leave behind contaminants such as metal oxides, debris, and iron, compromising the metal’s natural ability to resist corrosion. These materials can rust and corrode easily and so passivation becomes necessary.
Chemical passivation in stainless steel enhances the chemistry of the passive layer by increasing the ratio of stable chromium atoms to the more reactive iron atoms in the upper three to five atomic layers of the metal’s surface.
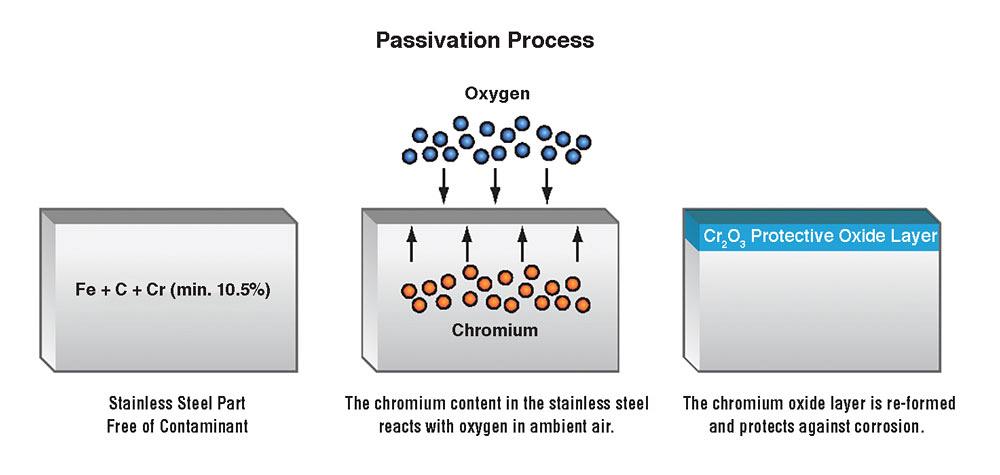
Removing the surface iron brings up the other components of the alloy like chromium and nickel to the surface. These elements, on contact with air, react with oxygen to form an oxide layer. This oxide layer protects the underlying layers of metal from corrosion.
The passive oxide layer that often forms naturally also heals the metal surface if it is damaged, as the next layer of molecules will then bond with the environmental elements.
Stainless steel and aluminum are both self-passivating materials, but they are not impervious to corrosion. Variation in grain size, due to heat treatment during manufacture, can weaken the metal.

It is indeed counterintuitive but passivation creates corrosion on the surface. This creates a thin layer of stable compounds. This stable layer binds tightly with the metal below and creates a hermetic seal. The seal blocks the ambient elements from further corroding the metal. Metal passivation is complete when it covers the entire surface with a tightly bound layer of corrosion. Corrosion causes weakness in metal which can stress it to a point of failure. Metal passivation is an important method in the fight against corrosion.
Metal Passivation Process
There are three significant steps in the passivation process: cleaning, pre-rinsing, and acid treatment.
Cleaning
Contaminants such as free iron, dirt, grime, grease, etc., on the surface of an object, may impair the formation of the passive film. Therefore, removing these contaminants produces corrosion-resistant clean objects. This first step is critical in the passivation process.
The cleaning often takes place in a separate cell. The process is based on the type and amount of contaminants, the level of cleanliness required, and the object’s shape and size. It might involve a water spray jet, vapor degreasing, or immersion in some cleaning solution with mechanical agitation.
Pre-Rinsing
The pre-rinsing follows immediately after the cleaning process. Rinsing water with mechanical agitation improves rinsing significantly. Counter-flow rinsing techniques improve the use of water efficiently, ensuring conservation and reducing waste.
Acid Treatment
The acid stage of the passivation process follows next. This step involves dipping the metal objects in dilute nitric acid or citric acid. This step removes free iron and other contaminants that have survived the previous steps and helps in forming the natural passive layer on the metal making it corrosion resistant.
Traditionally, nitric acid was used to passivate stainless steel, but now most manufacturers prefer citric acid. Citric acid passivation has many advantages:
- Citric acid only removes iron and does not remove other elements in the alloy thereby limiting the depth of the protective layer.
- Using citric acid, the cycle time is shorter.
- Treatment with citric acid avoids discharging heavy metals in the waste stream.
- Citric acid is safer and environmentally friendly.
Final Rinsing
The final rinsing may be a combination of water spray and immersion method, depending on the size of the object and water management policy. Sometimes a dip in neutralizing solution of 5% sodium hydroxide or sodium dichromate may be necessary after rinsing. After the final rinse, the metal objects are quickly dried.
Standards and Quality
To ensure the quality of stainless steel and aluminum grades, there are now standard passivation processes and tests. For passivation for various industries including the military, ASTM standards A380 and A967 provide the guidelines for good passivating practices. These standards provide acceptable methods for passivation including details of tests for measuring the effectiveness of passivation.
Normally the passivation requires complete immersion of the objects in a passivation solution. The vessel should be large enough so that the solution can slosh around the objects, which can be further enhanced with recirculation or ultrasonic vibration. The concentration of the acid, temperature of the bath, and contact time are all are very critical to the successful implementation of the process.
The quality of the passivation process is evaluated by inspection and testing the passivated objects. Upon completion of the passivation process, the metal objects should show no surface anomalies or defects such as pitting or frosting.
Alternatives to Passivation
The most common alternative to passivation is….to do nothing. If metal is appropriately cleaned, handled, and coated, frequently there will not be a need for passivation.
One other method which is also quite popular is called pickling. Pickling also provides a metal surface chemical treatment to make the metal objects corrosion resistant. Compared to pickling, the passivation process is a milder process. According to ASTM A967, after pickling, passivation is not required. However, some customers request metal pickling and passivation as part of the treatment process.
Metals may require passivation for critical applications. Geographic examples include installations near the coast, offshore, or in severe-duty applications. Industries that commonly use passivation include aerospace and medical.



