Ejectors and eductors are liquid jet pumps that use water or other liquids or gas under pressure to create a pumping action. Operators frequently confuse ejector vs. eductor pumps due to their similar names and similar appearances.
In this article, you learn about the differences between an ejector vs. an eductor, typical applications, and other ways to discharge a cavity.
Differences of Ejector vs. Eductor
Function
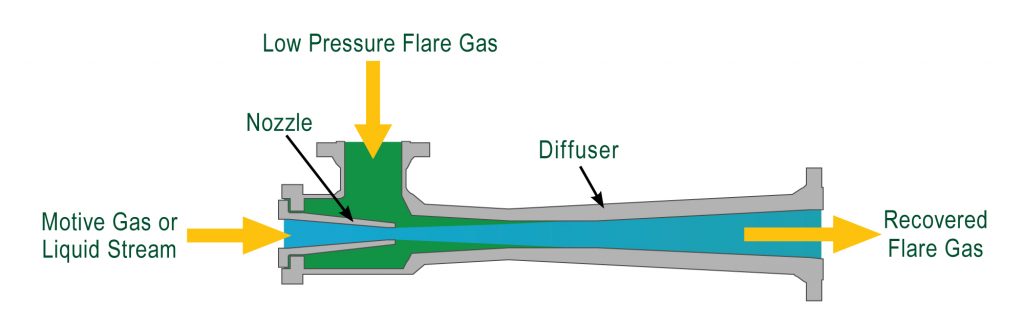
An ejector is a type of vacuum pump, which produces a vacuum through the Venturi effect. Working fluid flows through a jet nozzle into a tube that first narrows and expands in a cross-sectional area in an ejector. That fluid entrains and then compresses a low-pressure media to the required discharge pressure.
At first glance, an eductor operates nearly the same as an ejector. Further complicating this concern is physically, the eductor and ejector appear similar and at times identical.
However, their primary function gives a clue to the difference. Ejectors maintain a system vacuum for a particular processing purpose whereas the eductor’s main objective is to remove fluid out of a system. To do so, ejectors pump gas while eductors pump liquids.
As seen from the table below, in general eductors usually have a larger form function due to their need to pump liquids vs. gaseous substances. Since eductors pump liquids, velocities of the pumped liquids are much lower vs. ejectors.
| Attribute | Eductor | Ejector |
| Process Media | Liquid | Steam or Air |
| Nozzle Type | Converging Type | Converging-Diverging |
| Suction Head Size | Oversized | Smaller Than Eductor |
| Operating Velocity | ~10 ft/sec | ~1,000 ft/sec |
| Bore Size Limit | No Limit | Limited to small diameter |
| Noise | Low dB | Low to Medium dB |
| Compression Ratio | High | Low |
Common Applications

Eductor
- Sampling: Process gas sampling and aspiration for continuous analysis or discrete sample for lab analysis are possible with in-process analytics. The piece can also be returned to the process using the eductor.
- Mixing or dilution: The mini-eductor can be used to mix samples in a specific controlled ratio. Thus, any pressurized gas (air, nitrogen, argon, natural gas, etc.) can be used as the motive force that creates the vacuum flow.
- Vacuum: Creates a vacuum for evacuating vessels or lines.
- Venting: Remove harmful vapors from a chemical process or vessel.
- Eductor on Ships – Creating a vacuum in a freshwater generator, also in vacuum toilet systems, as a self-priming system for centrifugal pumps, foam applicator in fire extinguishing system, as well as other processing applications.
Ejector

- Crude oil distillation – Ejectors see frequent use in crude vacuum distillation, lube oil distillation, vacuum flashing, and desulfurization applications.
- Petrochemical processes – Petrochemical processes are vacuum intensive. Thus, depending on the required plastic to be manufactured, single, two, three, and four-stage ejector systems may be appropriate.
- Edible oil deodorization – The food industry benefits from the use of food-grade ejector systems. Thus, common applications include its use in edible oil plants for deodorization. Also, other ejector applications in edible oil refineries include vacuum bleaching, hydrogenation, deaeration, and interesterification.
Other Ways to Discharge a Cavity
Gravity Feed
Gravity feed is a method of moving anything (typically a liquid) from one location to another using the Earth’s gravity. Thus, this manual method makes sense for small quantities of discharge or manual batching. For example, typical application is fuel supply to an internal combustion engine by placing the fuel tank above the engine.
Even if a more sophisticated method is specified, gravity feed considerations play into all pump provider’s calculations. Gravity effectively provides the NPSHa calculation.
Osmosis
Osmosis is the net movement or diffusion of solvent molecules from a location of high water potential to a region of low water potential through a selectively permeable membrane. Thus, this process equalizes the solute concentrations on both sides.
The water (or another solvent) must travel because the solute particles cannot pass the barrier. Osmosis is thermodynamically advantageous because the closer the system approaches equilibrium, the more stable it becomes.
Phase Change
Boiling
Boiling is a phase transition from the liquid to the gas phase at or above the boiling point. When a liquid is heated to the boiling point, it undergoes rapid vaporization.
Heating
Substances that change phase absorbs and release thermal energy are phase change materials (latent heat). When a substance melts, it transitions from solid to liquid. Because different materials melt and solidify at different temperatures, they absorb different amounts of heat energy.
Condensation
Condensation converts a substance’s vapor phase to its liquid phase by removing and transferring heat from the vapor phase to a cooling medium. Also, for the process to occur, steam must be saturated, and the warmth of vaporization must be removed from the fog.