A saturated liquid is a fluid that is about to boil. It has reached a state where any decrease in pressure without a temperature change would initiate boiling.
In this article, you’ll learn more about saturated liquid properties, its properties vs. saturated steam, vs. compressed liquid, and examples.
Saturated Liquid Properties
Some thermodynamic properties are measured easily, whereas others derive using relationships between them and quantifiable qualities. The results of these computations and measurements are available in tables in an easy-to-understand format.
The qualities of a saturated liquid denote as subscript “f,” and soaked vapor presents as “g”. The difference between the same characteristic’s soaked steam and saturated liquid values denote by another subscript, “fg.”
where:
- vf = specific volume of saturated liquid
- vg = specific volume of saturated vapor
- vfg = difference between vg and vf ( vfg = vg – vf)
- Enthalpy: is a property define as H = U + PV (kJ) or h = u + Pv (kJ/kg) (per mass unit).
- The amount of energy that requires to evaporate a unit mass of “f” at a given temperature or pressure, called enthalpy of vaporization (or latent heat). As the temperature or pressure rises, it lowers until it reaches zero at the critical point.
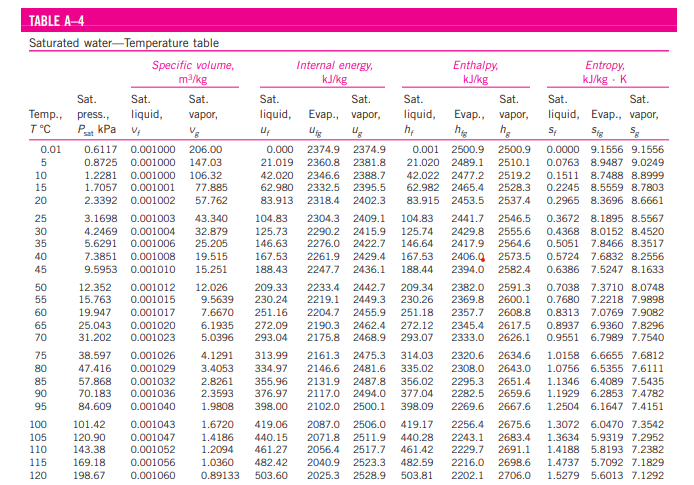
Look in Table A-4. In the row corresponding to the value of T, find the specific volume of (vf) and the particular importance of saturated vapor (vg).
If (v = vf), then the fluid is saturated liquid, and the values of pressure, internal energy (uf), enthalpy (hf), and entropy (sf) is read directly from the row corresponding to the known temperature T.
Saturated Liquid vs. Saturated Steam
The saturated liquid is fluid that is on the verge of boiling. Its vapor pressure has equaled total environmental pressure, but it remains in a liquid state. It will start boiling at any moment. It is a momentary stage in phase change.
For example, water at 100°C at 1 atm pressure in the fluid state is saturated.
Saturated steam is the complete reverse of the above, it is the vapor that is on the verge of condensing.
For example, steam at 100°C and 1 atm pressure is a perfect example of this.
Therefore, the (vf) is at the boiling point, while soaked steam is steam at the dew point. For water at a given pressure, the liquid and vapor phases are both at the same temperature. And steam contains a higher enthalpy, with the difference between that and the liquid being the latent heat of vaporization at the given pressure.
Saturated Liquid vs. Compressed Liquid
Saturated liquid can’t have any kind of solute added to it at that temperature. In addition, it can alternatively characterize as a form of liquid in which solutes can no longer dissolve at this temperature range due to the lack of intermolecular gaps (empty spaces between molecules). This liquid begins to boil if the pressure drops or if further heat applies.
On the other hand, a compressed liquid means a fluid under mechanical or thermodynamic conditions that force it to retain a liquid phase. Unlike the saturated liquid, the compressed liquid does not need to be at the exact state of phase change. As a result, a compact liquid’s actual pressure and temperature provide more significance than its saturation pressure and temperature
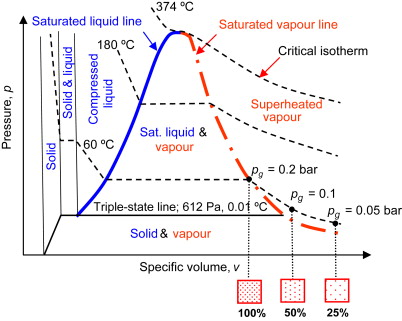
Compressed liquids also are referred to as subcooled liquids when temperature provides the mechanical attribute that forces it into the liquid phase. For compressed liquids, any of these attributes can cause a compressed liquid to retain its state: specific volume and enthalpy lower than a saturated liquid, temperature below the saturation point, and pressure above the saturation pressure.
Saturation Examples
Saturated liquids typically become of use during heat transfer applications. Most frequently, their phase state and corresponding thermodynamic properties find value in air conditioning and heat dissipation applications. Chemical engineers use their properties during the PFD design process.
The saturation state of various liquids at atmospheric pressure is as follows:
- Water – saturation condition of the water is 100°C
- Carbontetrachloride (R10) – Temperature 76.69°C
- Ammonia – Temperature -33.33°C
- Ethylene glycol – Temperature 197°C
- Petrol – Temperature above 37.5°C
- Acetone – Temperature 56.7°C
- Methyl alcohol –Temperature 65°C
- Ethyl alcohol – Temperature 77.8°C
- Kerosine – Temperature above 151°C
This saturation state also indicates the subject liquid’s boiling point. Therefore, the saturation state provides the temperature where fluid vaporizes with a slight increase in heat. Thus, the saturation temperature of the watery fluid is also called the boiling point of that liquid.



