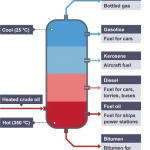
At atmospheric pressure, diesel has an initial boiling point of 392°F (200°C) and a final boiling point of 662°F (350°C). This wide range is due to its variety of blends which alter its boiling point value. Pressure is another factor that alters diesel’s boiling point. In this article, you will learn the blends and compounds in diesel, how they affect its boiling point, and pressure’s effect on diesel’s boiling point.

Blends and Compounds in Diesel
Diesel fuel, similar to gasoline, is a product via fractional distillation of petroleum. In its raw state, diesel fuel contains more nonvolatile components than gasoline, resulting in a higher boiling range. Diesel is required to be sprayed and cannot be evaporated in the air without the risk of pre-ignition. To solve this problem, diesel fuel is mixed with many compounds creating different blends to adjust performance.
Most compounds added to diesel fuel are hydrocarbons of three main classes: paraffinic, naphthenic, and aromatic. It also contains small amounts of organic compounds such as sulfur, nitrogen, and oxygen. These substances are distributed uniquely to make different grades/types of diesel fuel.
Diesel #1 vs Diesel #2
Diesel #1 contains fewer energy components and is harder to produce when compared to diesel #2. Because of the increased production difficulty, diesel #1 tends to be more expensive when compared to its rival. It performs better in colder temperatures due to the removal of paraffin allowing the fuel to always remain in liquid form, and is often referred to as the ‘winter diesel’.
Diesel #2 is the grade you will most likely find at your local gas station. It contains a higher number of energy components and lubricating properties, resulting in overall better fuel performance. It is easier to produce when compared to #1, making it less expensive. The only downside of diesel #2 is that in cold temperatures, the fuel tends to thicken into an almost gel-like state that can cause hard starts and stalling. Despite this, diesel #2 works year-round in many climates and locations.
How Blends and Compounds Affect the Boiling Point
Diesel fuel in general has a higher boiling range than its gasoline counterpart. Looking at diesel #1 vs diesel #2, their unique properties and makeup cause a difference in the boiling point.

Diesel #1 vs Diesel #2 Boiling Point
The ‘winter diesel’, or diesel #1, makes up for the low end of diesel fuel’s boiling point, varying anywhere from 390°F to 525°C. Because of the added substances that make the grade perform better in cold weather, it results in lower boiling points and worse performance during summer.
Diesel #2 has a high boiling point and is often referred to as the ‘summer diesel’ for its good performance in the summertime. It accounts for the top of diesel fuel’s boiling range and tends to be anywhere from 540°C to 660°C.
The ‘Gelling’ Point
As stated in the section above, the main difference between the two grades is performance in cold weather conditions. The ‘gelling’ point of diesel fuel is the temperature at which the fuel begins to thicken up into a gel-like solid form, causing vehicle ignition problems. Diesel #2 is often not used in the cold due to its high gelling point of around -7°C (19°F). Contrary, diesel #1 has a gelling point of -40°C (-40°F) and is the preferred option for winter.

Pressure’s Effect on the Boiling Point
As mentioned above, each diesel blend consists of a unique amount of hydrocarbons and organic compounds. Differing amounts of each will therefore have a direct influence on the vapor pressure of the blend as a whole. Hydrocarbons are volatile organic compounds that have low boiling points and evaporate quickly into the air. Blends containing a high amount of hydrocarbons will have boiling points in the lower range and vice versa. Many countries have placed limits on the number of hydrocarbons allowed in blends due to their classification as heavy pollutants.
Comparing vapor pressure to the boiling point, you will find that the two are indirectly related to one another. Volatile blends with heavy hydrocarbon composition will have low boiling points, producing more gas and vapor pressure. Non-volatile blends with more organic compounds in their makeup will have higher boiling points and produce less gas and pressure. The inconsistent vapor pressures of diesel blends lead to a wide range of boiling points.