Hydrogen induced stress cracking refers to cracking in steel due to the buildup of hydrogen molecules in its microstructure. In this article, you will learn more about hydrogen induced stress cracking, testing for it, and the influence of welding.

More on Hydrogen Induced Stress Cracking
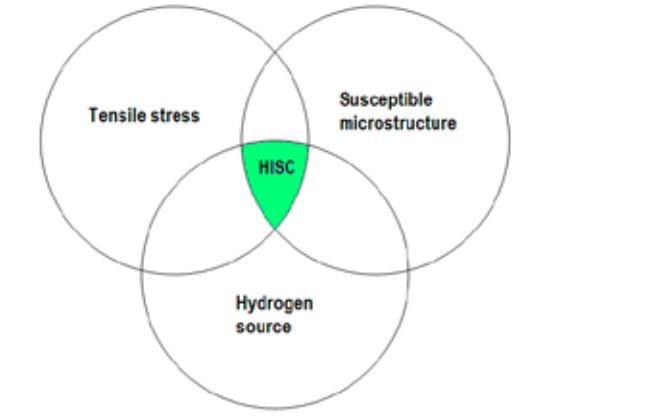
Hydrogen induced stress cracking (HISC) is a phenomenon that is common with low-alloy steels and high strength steels. Because of the microstructure of these metals, it is easy for hydrogen atoms to permeate and lodge within their lattice. So, when these metals experience corrosion from acids such as wet hydrogen sulfide and hydrofluoric acid, the hydrogen atoms that are liberated end up within the metals. Over time, these atoms buildup thereby increasing the pressure within the lattice structure which leads to crack formation. On the application of tensile loads, the metal piece could end up failing at stress levels far below its SMYS. This is why hydrogen induced stress cracking is also referred to as hydrogen embrittlement. For this phenomenon to occur, there are three basic components, which are tensile stress, susceptible microstructure, and a hydrogen source.
Apart from corrosion, utilizing impressed current cathodic protection is another factor that can contribute to embrittlement, if not properly managed. Cathodic protection parameters such as overpotential, current density, and charging time can affect hydrogen absorption. Moreover, experiments show that the more negative the cathodic potential, the higher the absorption of hydrogen. For current density, an increase in it leads to more hydrogen in steels. Whereas the relationship between charging time and hydrogen content in steel is more complex. Studies show that there is a rising trend, then a decreasing trend with increasing charging time.
Hydrogen Induced Cracking Tests
Hydrogen induced cracking tests aim to assess the susceptibility of a manufactured part to HISC. Mostly, it is carried out on carbon and low alloy steel that are in wet sour service (H2S). For the test to be effective a specimen should have the same or greater hardness level than the actual part. Some of these tests are as follows:
- Testing without loading: Some standard tests check for occurrence of hydrogen induced cracking based on hydrogen absorption only. One of which is the NACE TM0284. This test entails exposing an unstressed specimen to the specified environment with hydrogen sulfide gas at 1-bar for 96-hours. Alternatively, testing could involve using partial pressures of hydrogen sulfide for up to 30-days. After the exposure period, measuring parameters such as crack sensitivity ratio, crack thickness ratio, and crack length ratio reveal the specimen’s resistance to HISC.
- Stress-oriented hydrogen induced cracking tests: These are tests that evaluate the resistance of steel to through-thickness cracking while under tensile stress. Various standards cover different methods such as BS 8701 (Full Ring Test), NACE TM0316 (Four Point Bend), and NACE TM0103 (Tensile Test).
- Evaluation of coating and plating: Sometimes equipment have coating or plating to prevent corrosion or improve aesthetics. So, it is necessary to assess the susceptibility of these coating or plating layers to HISC using standards such as ASTM F1940, ASTM F519, and ASTM B839.
HISC in Welding
Welding is another activity that can result in hydrogen induced stress cracking in steel. It also referred to as “cold cracking” because it occurs at room temperature, after cooling of the weld. Generally, it initiates at the heat affected zone (HAZ) or weld bead, before spreading to other areas. Factors that influence it are weld metal hydrogen content, parent material composition and thickness, heat input, and stresses acting on the weld.
Weld Metal Hydrogen Content
The higher the weld metal hydrogen content, the more the risk of stress cracking. Primarily, the source of hydrogen is moisture in the flux. This could stem from the flux in cored wires or flux used in submerged arc welding. Also, the amount of hydrogen generated depends on the type of electrode, with basic electrodes generating less hydrogen than its cellulosic and rutile counterparts. Other sources of hydrogen during welding are rust, cleaning fluids, paint/coatings, oil, grease, and dirt.
Parent Material Composition
The composition of the parent material influences its hardenability. Usually, this hardenability is estimated using a parameter called the carbon equivalent (CE) value. Higher CE values mean higher cooling rates, thus, increasing the risk of forming a hard brittle structure in the HAZ. Generally, steels with CE value less than 0.4 are not susceptible to hydrogen induced cracking if using low hydrogen welding consumables and processes.
Parent Material Thickness
Thickness of the parent material influences the cooling rate and by extension, microstructure of the HAZ, the hardness level, and the hydrogen retained in the weld. The thicker the material, the slower the cooling rate and the lower the risk of hydrogen stress cracking. Aggregating the thicknesses of material meeting at the joint line is how to quantify the thickness value. As a result, a fillet weld has greater risk than a butt weld for a material of the same thickness.
Heat Input
The higher the heat input, the lower the risk of HISC in the HAZ. However, if the diffusion distance for the escape of hydrogen from the weld bead increases with increasing heat input, then cracking risks increase.
Stress on the Weld
Locations of stress concentration such as the root and toe of the weld are likely points of crack initiation. This is why preheating and post-heating to minimize stress around the weld helps to avoid HISC.
Identifying and Preventing Hydrogen Stress Cracking
On most occasions, hydrogen stress cracking occurs at the steel surface, so are easy to identify using surface NDT methods. Sometimes these cracks are even visible to the naked eye, especially when they traverse across the pipe of a weld cap. NDT methods such as magnetic particle inspection, dye penetration test, and ultrasonic test are effective in identifying and quantifying HISC.
However, it is best practice to take all necessary measures to prevent HISC. Because regular inspection may not be possible, particularly for remote parts. Most prevention methods entail minimizing contact between the metal and hydrogen, but it is necessary to avoid procedures such as acid pickling. If possible, heat treatment such as low hydrogen annealing could serve to diffuse out hydrogen. In the case of welding, pre-heating and post-heating treatments take out whatever hydrogen absorbed in the HAZ. As this may not be possible in many applications such as subsea pipelines, it is best to prevent HISC by material selection. To help with this, tests like ASTM F1624 provide a means of ranking alloys to ensure that the cracking threshold for the material is below that of HISC.