Generally, the term ‘flexible’ is not technically accurate for describing the characteristics of metals. Rather, a flexible metal is one that combines two key metallic attributes, which are malleability and ductility. In this article, we discuss the most flexible metals in commercial use, as well as why some metals are more flexible than others.
The Most Flexible Metals
Ductility refers to the ability of a metal to undergo plastic deformation under tensile stress without rupture/failure. A metal exemplifies its ductility by being drawn into a wire without losing strength or breaking. On the other hand, malleability is the ability of a metal to undergo plastic deformation under compressive stress without rupture. Moreover, malleability quantifies a metal’s ability to be beaten into sheets. Although both characteristics appear similar, ductility in any metal does not necessarily equate to malleability, and vice versa. For example, lead can undergo significant compressive stress, but fails easily under tension. So, for metals to be flexible, they need to have these two features in significant measure.
Gold

Gold offers a perfect combination of ductility and malleability, which is why it ranks highest in the list of flexible metals. A single ounce can be drawn to a length of 50 miles. Similarly, an ounce of gold can be beaten to form a 187 ft2 gold leaf. These attributes are due to its microstructure and it being one of the densest metals at 19.3 g/cm3. As a result, gold serves effectively as premium wires and in other electrical applications, because it is a good conductor of heat and electricity. Its malleability enables its formation into various shapes and sizes for jewelry, electronics, medical implants, etc.
Aluminum

Aluminum is the most abundant metal on earth, often existing as oxides and salts due to its high chemical reactivity level. Like gold, aluminum has good ductility and malleability. Also, it has high strength-to-weight ratio and excellent resistance to corrosion, which makes it predominant in several applications. The low density and low melting point of aluminum contribute to its high ductility and malleability for a wide range of temperatures. Its features make it predominant in electrical applications for making terminals and contactors, but not common in wires, due to some pitfalls. They are excellent as sheet metals for vehicle bodies, roofing sheets, and cans for food storage.
Copper

Although copper is a heavy and dense metal, its face-centered cubic atomic structure allows for relative motion, without breaking bonds. This accounts for its good ductility and malleability, showing total elongation of up to 60% before fracture. In combination with its excellent electrical conductivity and relatively low cost, it is the number one choice for electrical wires globally. Its malleability also makes it a good choice for jewelry, cookware, pipes, and cladding.
Platinum

Platinum is a silver-colored, highly dense (21.5 g/cc) metal in its pure form. Also, it is rare and has several desirable physical properties, so quite expensive. Platinum is more ductile than gold, and arguably the most ductile of metals. It is highly resistant to corrosion with a boiling point around 3,220℉, and is one of the most stable elements in nature. Because platinum is immune to nitric and hydrochloric acids it commonly serves in laboratories as electrodes. It also serves in improving the efficiency of pacemakers, wires, and optical fibers. However, its most common application is as a catalytic converter in cars where it converts CO and other residual pollutants into CO2 and water vapor. As one of the most inert flexible metals, it is suitable for making exclusive jewelry.
Silver

Like platinum, silver is one of the lustrous flexible metals as it exhibits good ductility and malleability. Its decorative aesthetic makes it a popular material for jewelries, but it is soft so often alloyed with materials such as nickel and palladium. Because it has high thermal and electrical conductivity, alloying silver with copper delivers premium electrical wiring and conductors. This alloy has high ductility, being able to achieve up to 40% elongation in a tensile test.
Why Are Some Metals Flexible?
Generally, ductility and malleability are a common feature with metals due to their microstructure. Within each metal, atoms form a metallic bond, which allows electrons from atoms to move freely between each other. As a result, atoms are able to slide past each other to an extent, while maintaining this bond, hence allowing metals to stretch. So, why are some metals more flexible than others? One of the reasons is the number of valence electrons. It is the valence electrons that are able to move freely between atoms. Thus, the higher the number of valence electrons, the more flexibility a metal possesses.
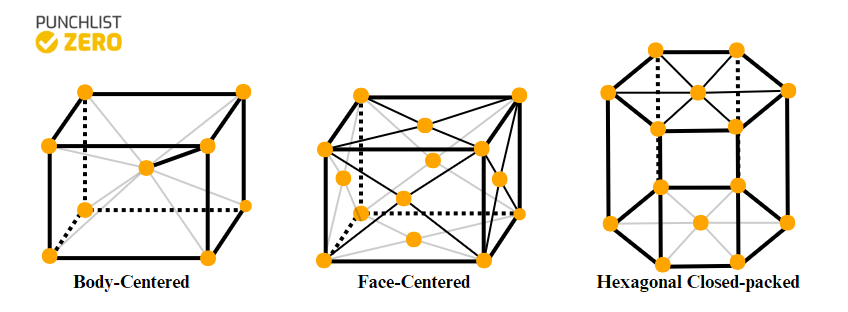
Another factor that makes some metals more flexible than others is the type of crystal lattice they have. Typically, metals have either face-centered, body-centered, or hexagonal closed-packed lattice structure in their solid state. When deformation of a metal occurs, it happens along the slip planes of its lattice structure. So, the more slip planes a lattice structure has, the more possibilities for deformation, leading to more ductility and malleability. Out of the common lattice structures, face-centered and body-centered lattices have the most slip planes. However, metals with face-centered lattice structures are the most flexible because their slip planes have a closer packing in comparison to body-centered lattice. Thus, requiring significantly lower energy expenditure during deformation.



