
Propane and propylene are both fuel gases that serve in a variety of applications. Propane is a constituent of natural gas with a chemical formula C3H8. Whereas propylene is a by-product of petroleum refining and has a chemical formula of C3H6. In this article, you will learn key differences between the two gases, the propane-propylene separation, and understand the propane vs propylene spread.
Differences Between Propane vs Propylene
Propane and propylene share several similarities including being gases at room temperature, due to their low boiling point. Also, both are flammable compounds that are easy to liquify, so they are ideal for LP gas. However, there are features that make them unique, which the following table highlights.
| Propane | Propylene |
| It is an odorless alkane and a constituent of natural gas (up to 5%). | Unlike propane, propylene is an alkene with a pale petroleum odor. Generally, it is obtained by cracking petroleum. |
| Propane is made up of 8 hydrogen atoms, has a molecular mass of 44.10 g/mol, and with a boiling point of -42 °C. | As for Propylene, it has 6 hydrogen atoms, with a molecular mass of 42.08 g/mol, and a boiling point of -47.6 °C. |
| Its carbon atoms have only single bonds, which makes it a less reactive gas. However, it reacts with halogens in the presence of sunlight. | Has both single and double bonds. Thus, giving it combustion advantage as it burns hotter. Also, the presence of double bonds gives it higher tendency to react in non-fuel applications. Some of these include polymerization, oxidation, halogenation, and hydration. |
| Its features make it ideal for space and water heating, cooking, crop drying, weed control, and powering equipment. | The superior combustion performance makes it efficient for heating, cutting, and as a high-velocity oxygen fuel. Also, useful for organic synthesis. |
Propane-Propylene Separation
The separation of propane and propylene is one of the most energy-intensive processes in the chemical industry. Largely because their pure component boiling points are close over a wide range of pressure, so they are difficult to separate by ordinary distillation. Nevertheless, this separation is a necessary step in the production of propylene which is increasing in popularity in the petrochemical industry. Generally, separation processes of propane and propylene are low-pressure distillation, high-pressure distillation, and distillation with heat pump.
Low-pressure Distillation
The low-pressure distillation method improves the relative volatility of propane and propylene. Consequently, this reduces the reflux ratio and theoretical trays of the distillation process, which is favorable. However, this results in the need for a specialized condensing agent to cool the top of the tower, as water will not suffice. After condensation, the top product can be compressed as a cryogen and vaporized at the bottom. The complexity of the method sometimes outweighs its benefits, leading to the adoption of other methods such as the high-pressure distillation.
High-pressure Distillation
High-pressure distillation to separate propane and propylene from hydrocarbons is a much simpler method than low-pressure distillation. Rather than use a specialized condensing agent to cool the top of the tower (above 104 °F), ordinary water can serve. While low pressure steam is enough to satisfy the temperature requirement of about 158 °F at the bottom of the tower. Although this method is less complex, the reflux ratio is very high, and the number of theoretical trays is large. However, adding a solvent such as aqueous acrylonitrile to optimize the process could overcome the downside of this method.
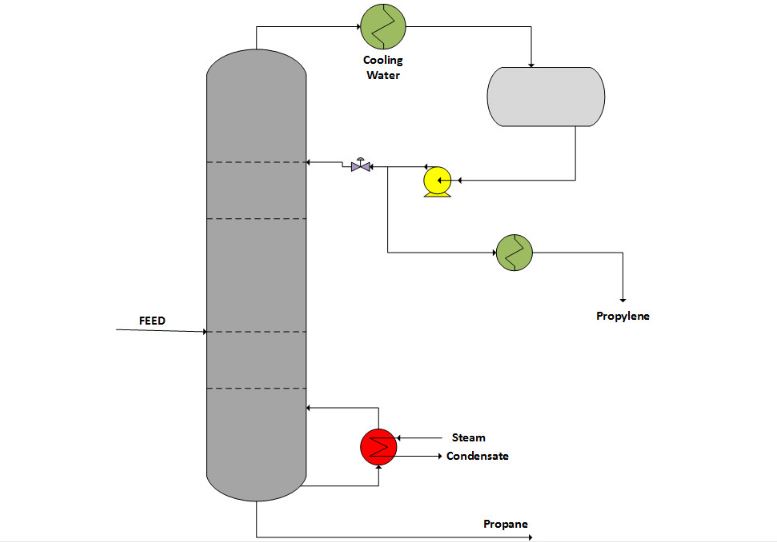
Distillation With Heat Pump
The introduction of heat pump technology to distillation enables the reduction of the operating pressure by up to 10 to 20 bar. This method uses the heat from the condensation of top products in the tower. As a result, the relative volatility of propane-propylene increases and makes it easier to separate. Also, there is a reduction in the reflux ratio and equilibrium stages or theoretical trays. To compensate for the idealities, it is necessary to add an auxiliary condenser with cooling water as the figure below shows.
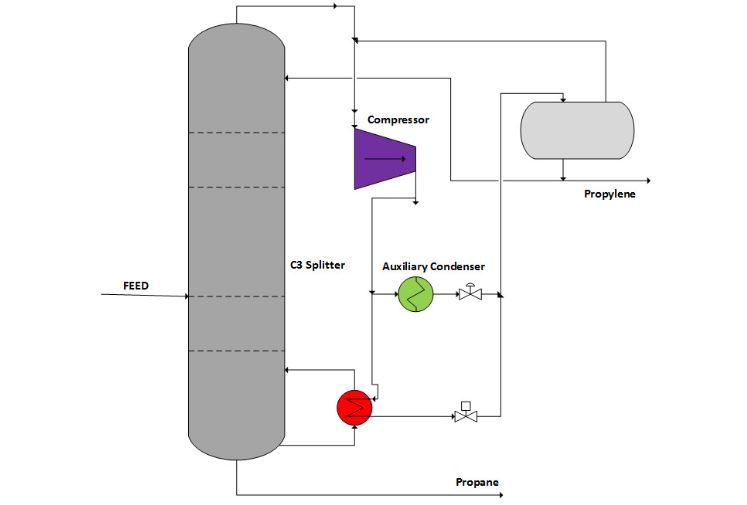
Due to the capital and energy intensive nature of distillation methods, alternative methods of separating propane and propylene include:
- Pressure vacuum swing adsorption.
- High quality carbon molecular sieve.
- Using a series of microporous hyper crosslinked polymers that are doped with silver.
- Also, catalytic dehydrogenation of propane in situations in which the propane value is low and derivatives are limited to local markets.
The Propane-Propylene Spread
Although propylene can be produced via a variety of means, the feedstock for its production is mostly propane. When this is the case, the difference between the price of the propane feedstock and the propylene selling price is the propane-to-propylene spread. Generally, propane prices are cyclical with peak prices during summer, while prices experience a deep during winter. On the other hand, propylene prices are controlled by its demand as well as the cost of production. Various methods of production deliver propylene at varying levels of purity, in line with the needs of end users. Also, the demand for propylene remains on the increase for use in the automobile sector, packaging material, and manufacturing of derivative chemicals. The spread of propane and propylene is always in flux and is a key parameter for producers of propylene. Because it determines the profitability of the procedure whenever propane serves as the feedstock.



