The refrigeration cycle is a specially designed thermodynamic cycle that removes heat from a closed environment to keep the inside cool. Likewise, it provides the exact opposite effect by simply reversing the cycle. This cycle occurs in almost every modern building through the HVAC systems and indoor refrigerators. In this article, you will learn the principles behind the refrigeration cycle, calculations, and applications.
Principles of the Refrigeration Cycle
The refrigeration cycle works very differently than most thermodynamic cycles that exist. Because of the thermodynamic laws that heat can only flow from hot to cold, The cycle seems like it should not be able to cool a space. However the refrigeration cycle works by instead taking the inside air, removing the heat from the air by transferring it to a refrigerant and then returning the cool air. Unlike other cycles the goal is to remove heat from an environment using electrical work rather than turning heat into mechanical work.
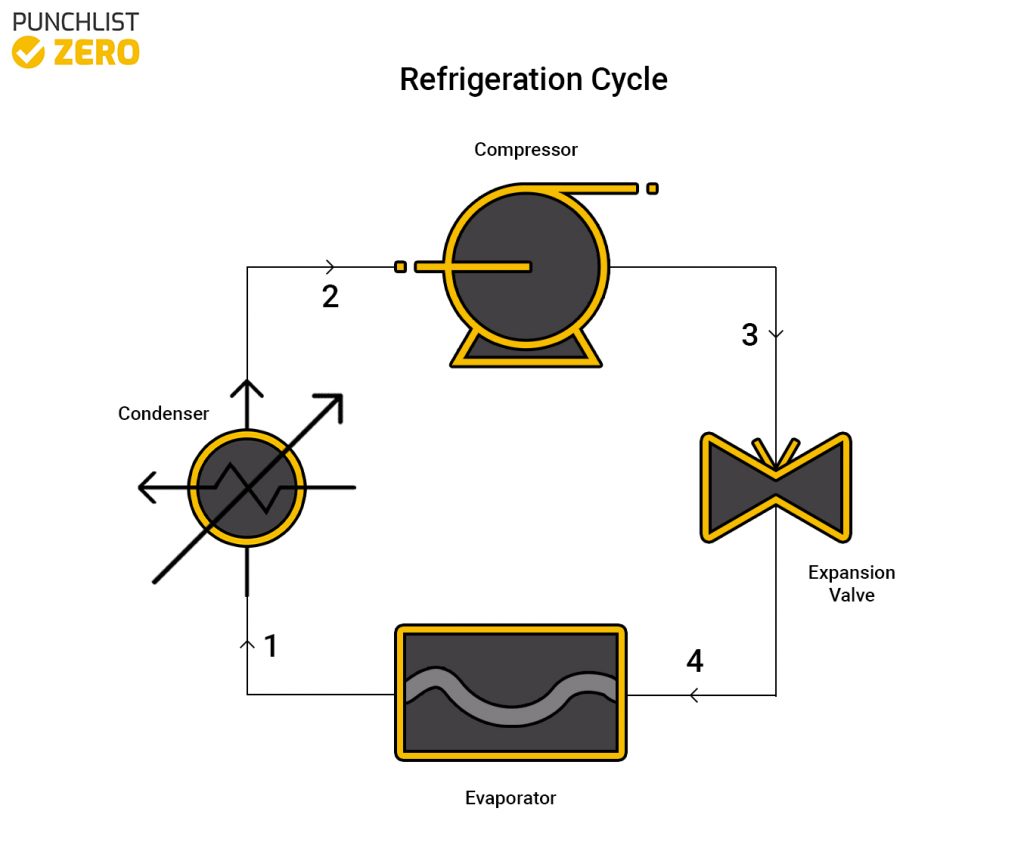
The refrigeration cycle consists of four main devices which work together to accomplish this goal. These four devices are a compressor, condenser, some sort of expansion device, and evaporator. The process begins by funneling some sort of refrigerant (commonly R-134), in gas form, through a compressor. The overall volume reduces and pressure raises, prompting a rise in temperature. Next, the now hot refrigerant enters a condenser to become a hot liquid. So that the liquid can expand by reducing the pressure using an expansion valve. Finally, the evaporator converts the cool refrigerant into a gas. The evaporator is the actual part of the cycle that does the cooling to the environment.
Calculations of the Refrigeration Cycle
To fully analyze the efficiencies and inner workings of a refrigeration cycle, the entropy and enthalpy require tracking throughout each stage. A pressure value for the condenser and the temperature of the evaporator needs defining as the baselines of the cycle. Finally, using refrigerant tables allows the determination of refrigerant properties of the refrigerant at different temperatures and pressures.
State 1 ➔ 2 (Condenser)
With the condenser entrance temperature defined, users may determine the enthalpy and entropy from the refrigerant tables. The quality, (ratio of vapor to liquid) f the fluid at this state equals 1. Thus, the enthalpy(h1 = hg) and entropy(s1 = sg) can be found.
Because this process is isentropic the entropy(s2 = s1) at state two is the same as at state one. Then, using the found entropy and the pressure leaving the condenser the enthalpy(h2) at state two can be found through interpolation.
![]()
State 2 ➔ 3 (Compressor)
The next step to analyzing the refrigeration cycle is the compressor part. Using the pressure from the exit of the condenser, the enthalpy(h3 = hf@P2) can be found.
State 3 ➔ 4 (Expansion Valve)
Because the process is throttling, the enthalpy(h4 = h3) remains constant throughout the expansion valve.
State 4 ➔ 1 (Evaporator)
With all the enthalpies determined, the inner workings of the given cycle can be fully analyzed. First and most importantly, the calculation of the cycle’s power may occur. The mass flow rate(ṁ) of refrigerant also provides for these calculations, and might need to be defined or measured to find.
![]()
Next, the calculation of cooling load determines how much cooling work occurs in the cycle.
![]()
Finally, we can use the cooling load and input power to find the Coefficient of Performance(COP). Usually, the COP falls between 2.3 to 3.5 and serves as a rating of how efficient a refrigeration cycle is.
![]()
Applicable Uses of the Refrigeration Cycle
The refrigeration cycle functions in many shapes and sizes throughout the globe. Almost every building in modern countries has some form of an air conditioner. Whether it is a window unit cooling an 80 sq ft room or a industrial-sized HVAC unit cooling an 16k sq ft warehouse. The commonly used cycle is incredibly useful and efficient at properly removing heat from an environment. These units are also variable in cooling power. For instance, with a fridge the average cooling temperature is around 40° F whereas a freezer averages around 0° F. Likewise, reversing the refrigeration cycle induces heating into an environment. The cycle’s flexibility to meet a variety of requirements means it is the most widely used for anything that needs cooling.
The cycle also serves other industrial uses. For instance, a form of the refrigeration cycle occurs in the pasteurization of milk. Another benefit of it is that it will actually dry things as well as remove heat.