Propane and butane are both forms of gases that are popular as fuel for commercial and residential use. This is largely because they are clean sources of energy compared to most fuel gas. They are also extremely versatile, being found in gaseous and liquified petroleum gas (LPG). Even in the case of fugitive emissions, they do not impact the atmosphere negatively as they are not greenhouse gases. Despite all these similarities, significant differences exist between propane and butane.
In this article, you will learn the differences between the two gases, review typical propane-butane ratios in LPG, and how to separate them.
Differences Between Propane vs Butane
| Parameter | Propane | Butane |
| Molecular Structure | It is an alkane consisting of three carbon atoms and eight hydrogen atoms (C3H8). | Also, an alkane but consists of four carbon atoms and ten hydrogen atoms (C4H10). |
| Boiling Point | Propane has a lower boiling point at -43.6 °C. | The boiling point of butane is 28.4 °C. |
| Vapor Pressure | Due to its lower boiling point, propane exerts about four times more vapor pressure than butane. So, it is ideal for external storage and applications in temperate regions. | It exerts lesser vapor pressure and turns to liquid much easier than propane. This limits its storage to indoor locations. Nevertheless, the lower vapor pressure makes it an effective propellant. |
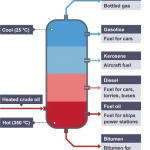
| Applications | Its features make it ideal for water heating, patio and home heating, BBQs, and LPG gas cooking. Also, it is a good fuel source for ovens, operating tools, and forklift trucks. | An ideal fuel source for portable heaters and an effective propellant for aerosols. |
Typical Rations in LPG
LPG, which is liquified petroleum gas, is a flammable mixture of hydrocarbon gasses. Moreover, it includes propane, butane, isobutane, propylene, butylene, and mixtures of these gasses. The percentage distribution of propane and butane in LPG varies according to season. Generally, propane takes up a larger proportion, if not 100%, of LPG during the winter season. As temperatures warm up in the spring or summer, the portion of butane in LPG increases. This is because the low boiling point of propane enables its storage and use as a gas in colder seasons.
Another factor that determines the propane-butane ratio in LPG is the location. For example, the term LPG gas in the US and Australia is not a mixture, rather it is 100% propane. On the other hand, the LPG mixture percentage is typically 60% propane and 40% butane in New Zealand. However, this percentage varies between its northern and southern islands during winter months.
How to Separate Butane from Propane
Because the boiling points of propane and butane are wide apart, they are much easier to separate vs propane and propylene. The process of separating both gases is a common step when distilling a stream of LPG into its constituents by passing it through a series of columns.
First, there is the separation of methane from other gasses in a high-pressure distillation column that operates at 180 K and 25 atm. Moreover, the column design is to keep a low concentration of methane in its bottom product. Next is a column otherwise known as de-ethanizer that operates around 264 K and 21 atm. This column serves for the removal of ethane from the hydrocarbon gas.
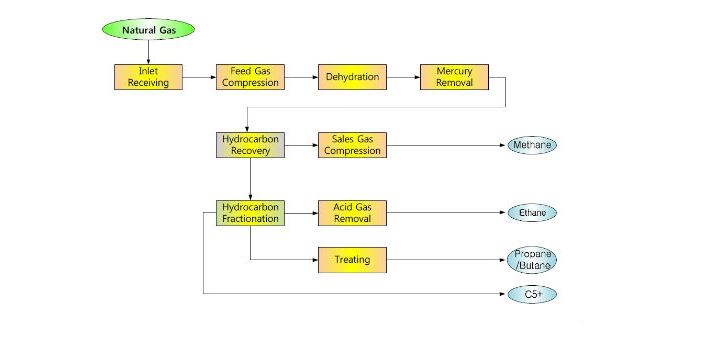
Subsequently, the feed passes through another column operating at 322 K and 17 atm to recover propane as distillate. In this column, the condenser uses cooling water and its design in a way that limits the concentration of propane impurity. Finally, a different column separates isobutane and n-butane after distilling other products. This is because these two gasses have the lowest relative volatility – their boiling points are very close. So, separating them in another column makes the process more economical. This final column operates about 322 K and 6.6 atm and has a design to separate isobutane as distillate, while n-butane is at the bottom.