The thinnest material known to man, graphene provides extreme strength, thermal conductivity, and electrical conductivity on par with copper. As such, this material has made an uproar with the scientific community and paved the way to mind-blowing applications such as bulletproof armor, graphene circuitry, ultra-light space crafts, healthcare and medicine, and even the construction of space elevators. In this article, you will learn about the properties of graphene, common applications, and production methods.
Properties
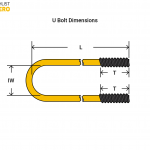
The commonly used material graphite is composed of millions of layers of graphene stacked onto each other. Graphene is a single layer of atoms arranged in a two-dimensional lattice. And this lattice produces a very strong yet flexible bond of carbon atoms. Thus, graphene retains the strength of diamonds with high bendability.
Due to the nanoscale of graphene layer, it is very light and much stronger than structural steel. It can also stretch up to 20% of its initial size without breaking, thus, making it flexible. The atoms in the material also delocalize electrons which makes it so good at conducting electricity and heat.
Common Applications
Common applications include mechanical reinforcement, electronics, energy storage, and anti-corrosion capability.
Mechanical Reinforcement
Graphene enhances the strength of materials such as plastics, rubber, steel beams, and other materials. By blending it with the subject material, high-temperature capabilities, and high tensile strength result. Due to its ultra-lightweight, vehicle production and aerospace applications such as aircraft and space ships are common applications. Product designers also blend the material into everyday items such as tennis rackets, bike wheels and frames, sunglasses, and even mobile phone parts.
Electronics
Graphene’s two-dimensional lattice lets electrons pass through them at the speed of light. This makes it potentially useful to replace silicon-based transistors which will make PLCs and computers a lot faster at handling and processing data. Overall, electronics should be able to operate at high frequencies increasing computational capacity, although commercial applications are still at the nascent stage.
Energy Storage
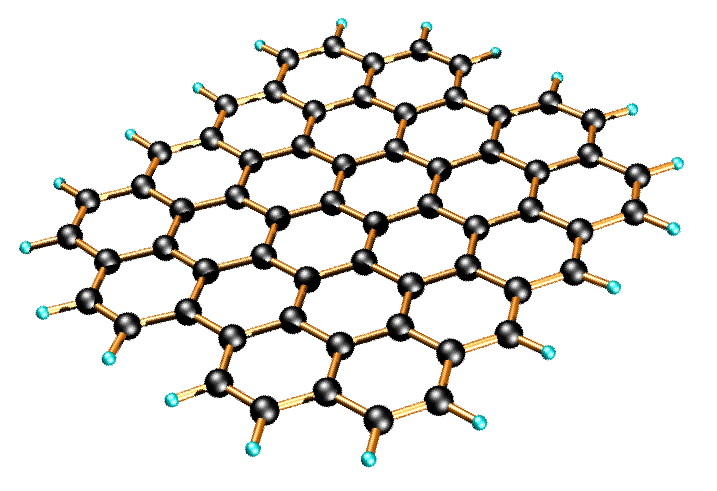
Another application of graphene is that it can be used as superconductors. Superconductors are a substance that conducts electricity without resistance. Since electrons move freely through the material, it is a potential material to use in batteries which makes them store more energy and charge faster too. Graphene batteries provide the same operating principle as traditional batteries with two electrodes and an electrolyte solution. The main difference lies in the material composition of the graphene-infused cathode.
Thermal Conductivity
Aside from being incredibly strong and lightweight, graphene provides great thermal conductivity. It can be a great material for heat spreading solutions such as heat sinks and heat dissipation films. Sheets of the substance provide ultra-thin touch screens, lightweight solar panels, and long-lasting LED lights.
Anti-Corrosion Capability
The impermeable structure allows for blending in paint or primers and with just one coating to prevent rust and corrosion. Applications in aluminum protection have shown there’s an optimal amount of graphene to be added to the coating. Too high of a concentration actually reduces overall corrosion resistance as a highly conductive circuit forms.
Production Methods
In 2004, Andre Geim and Konstantin Novoselov at the University of Manchester discovered and characterized the material graphene. They created it by using only graphite and sticky tapes. They placed graphite into the tape and folded it in two, and then cleaved the flake in half. After repeating this process a number of times they produced graphite with one single atom of thickness. This ultra-thin graphite became known as graphene. In 2010, Geim and Novoselov received the Nobel Prize for Physics for their discovery.
Today many other production techniques have evolved. Some, such as the commonly used flame synthesis method for nanoparticles do not adapt well to graphene production. Other major production methods include direct liquid-phase exfoliation, oxidation and exfoliation of graphite, chemical vapor deposition, and pulsed laser deposition.
Direct Liquid-Phase Exfoliation of Graphite
Liquid phase exfoliation is a type of mechanical exfoliation. Mechanical exfoliation includes methods such as stirring, shearing forces, ball milling, and using ultrasound on graphite suspended in a liquid. This method generally is not suitable for large-scale production.
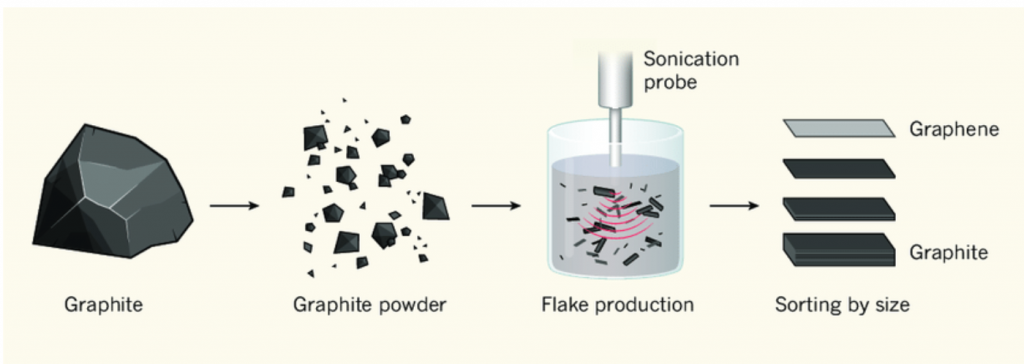
Oxidation of Graphite and Exfoliation and/or Reduction
Another approach to exfoliation is through the use of oxidizing agents such as sulfuric and nitric acids. The addition of acid oxidizes graphene sheets by forcing oxygen atoms between the sheets of graphene thus causing them to split apart. The resulting graphene oxide sheets suspended in acid and water and when filtered, leave flakes of graphene oxide. The final step removes oxygen and leaves a final product of pure graphene coating. This resulting material is called reduced graphene oxide or RGO.
Chemical Vapor Deposition (CVD)
In this approach, a chamber filled with gas such as methane heats a copper plate to 1000 degrees Celsius (1832 F) The methane gives up carbon atoms to the carbon plate, and the graphene self assembles itself atom by atom onto the copper plate. This method takes a lot of time to produce a small amount sheet and provides a lower quality outcome than exfoliation methods.
Pulsed Laser Deposition (PLD)
PLD provides a laser energy source outside of a chamber and the chamber maintains an ultrahigh vacuum. Material deposits via stoichiometry transfer on a target surface. PLD provides a low-temperature growth rate and thus high-quality graphene.